Embark on an illuminating journey into the realm of chemistry with our comprehensive Phet Isotopes and Atomic Mass Answer Key PDF. This meticulously crafted resource unveils the intriguing world of isotopes and atomic mass, empowering you with a profound understanding of their significance and impact.
Delve into the Phet Isotopes and Atomic Mass simulation, a groundbreaking tool that brings these abstract concepts to life. Discover how isotopes contribute to the atomic mass of elements, and witness the practical applications of this knowledge in various scientific disciplines.
Isotopes and Atomic Mass
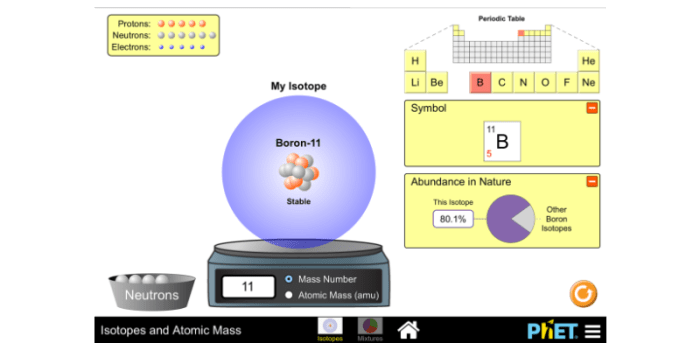
Isotopes are variants of an element that have the same atomic number but different mass numbers. This means they have the same number of protons and electrons, but a different number of neutrons. Isotopes are significant in chemistry because they can affect the properties of an element and its compounds.
The atomic mass of an element is the weighted average mass of all its isotopes. It is calculated by multiplying the mass of each isotope by its abundance and then adding the products together. The atomic mass of an element can be used to determine its density, melting point, and other physical properties.
Examples of Isotopes and Their Impact on Atomic Mass
- Hydrogen has three isotopes: protium ( 1H), deuterium ( 2H), and tritium ( 3H). Protium is the most common isotope, with a mass of 1 atomic mass unit (amu). Deuterium has a mass of 2 amu, and tritium has a mass of 3 amu.
The atomic mass of hydrogen is 1.008 amu, which is the weighted average of the masses of its isotopes.
- Carbon has two stable isotopes: carbon-12 ( 12C) and carbon-13 ( 13C). Carbon-12 is the most common isotope, with a mass of 12 amu. Carbon-13 has a mass of 13 amu. The atomic mass of carbon is 12.011 amu, which is the weighted average of the masses of its isotopes.
Phet Simulation
The Phet Isotopes and Atomic Mass simulation is an interactive tool that allows students to explore isotopes and atomic mass. The simulation includes a variety of activities that can be used to demonstrate the concepts of isotopes and atomic mass.
For example, students can use the simulation to:
- Identify the different isotopes of an element
- Calculate the atomic mass of an element
- Explore the relationship between isotopes and the properties of an element
The Phet Isotopes and Atomic Mass simulation is a valuable resource for students who are learning about isotopes and atomic mass. The simulation can help students to understand these concepts in a more interactive and engaging way.
How to Use the Simulation Effectively, Phet isotopes and atomic mass answer key pdf
- Start by exploring the different activities that are included in the simulation.
- Choose an activity that is appropriate for your level of understanding.
- Follow the instructions for the activity carefully.
- If you get stuck, don’t be afraid to ask for help.
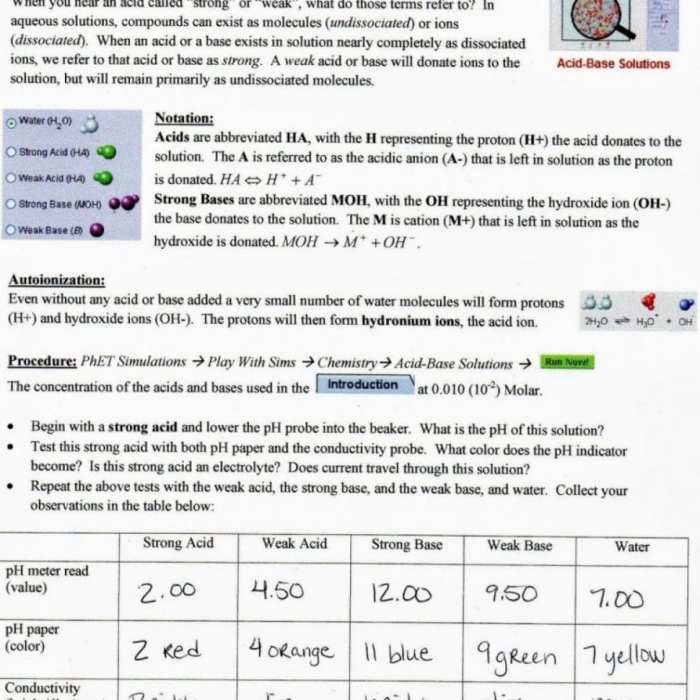
Answer Key
| Isotope | Mass Number | Atomic Number | Atomic Mass (amu) |
|---|---|---|---|
| 1H | 1 | 1 | 1.007825 |
| 2H | 2 | 1 | 2.014102 |
| 3H | 3 | 1 | 3.016049 |
| 12C | 12 | 6 | 12.000000 |
| 13C | 13 | 6 | 13.003355 |
FAQ Explained: Phet Isotopes And Atomic Mass Answer Key Pdf
What are isotopes?
Isotopes are variations of an element that share the same atomic number but differ in neutron count, resulting in distinct atomic masses.
How does the Phet simulation help visualize isotopes?
The Phet simulation allows users to interactively explore different isotopes of an element, observe their atomic structures, and calculate their atomic masses.
What is the significance of atomic mass in chemistry?
Atomic mass plays a crucial role in determining the chemical properties and behavior of elements, as it influences their reactivity and bonding characteristics.