The synthesis of ch3oh from co and h2 – The synthesis of methanol (CH3OH) from carbon monoxide (CO) and hydrogen (H2) is a crucial industrial process that has revolutionized the chemical industry. This reaction, known as methanol synthesis, plays a pivotal role in producing various essential chemicals, fuels, and solvents.
The process involves the catalytic conversion of CO and H2 into CH3OH, a versatile and widely used compound. Understanding the intricacies of methanol synthesis, from the chemical reactions to the industrial applications, is vital for advancing sustainable and efficient chemical production.
1. The Methanol Synthesis Process
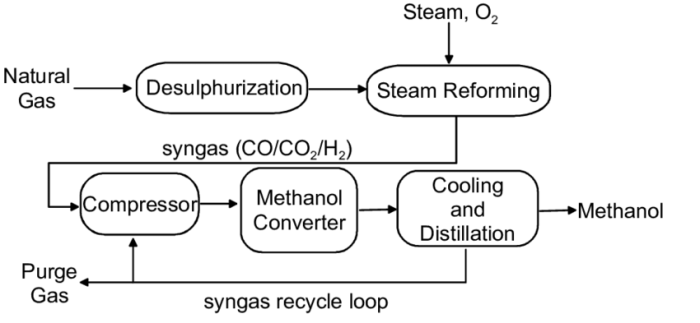
The methanol synthesis process involves the catalytic conversion of carbon monoxide (CO) and hydrogen (H2) to produce methanol (CH3OH). The reaction is exothermic and proceeds via a series of steps, including CO hydrogenation, formaldehyde formation, and methanol formation.
Industrial Process for Methanol Synthesis
The industrial process for methanol synthesis typically operates at elevated temperatures (200-300°C) and pressures (50-100 bar). The feedstock gases, CO and H2, are mixed in a specific ratio and passed over a heterogeneous catalyst, typically composed of copper, zinc, and aluminum oxides.
The catalyst promotes the reaction and selectively converts the reactants to methanol.
Key Factors Influencing Process Efficiency and Selectivity
- Catalyst composition and structure
- Reaction temperature and pressure
- Feed composition and purity
- Reactor design and operating conditions
2. Catalysts for Methanol Synthesis
Types of Catalysts
The most commonly used catalysts for methanol synthesis are copper-based catalysts, which are highly active and selective for the reaction. Other types of catalysts, such as zinc-based and iron-based catalysts, have also been investigated but are less widely used.
Catalyst Characteristics and Properties
- Copper-based catalysts:High activity and selectivity, low cost, and relatively stable.
- Zinc-based catalysts:Higher activity than copper-based catalysts but less selective and more prone to deactivation.
- Iron-based catalysts:Less active and selective than copper-based catalysts but more resistant to sulfur poisoning.
Role of Promoters and Support Materials
Promoters, such as zinc and aluminum oxides, are added to copper-based catalysts to enhance their activity and stability. Support materials, such as silica and alumina, provide a high surface area for the catalyst and improve its mechanical strength.
3. Reactor Design and Operation
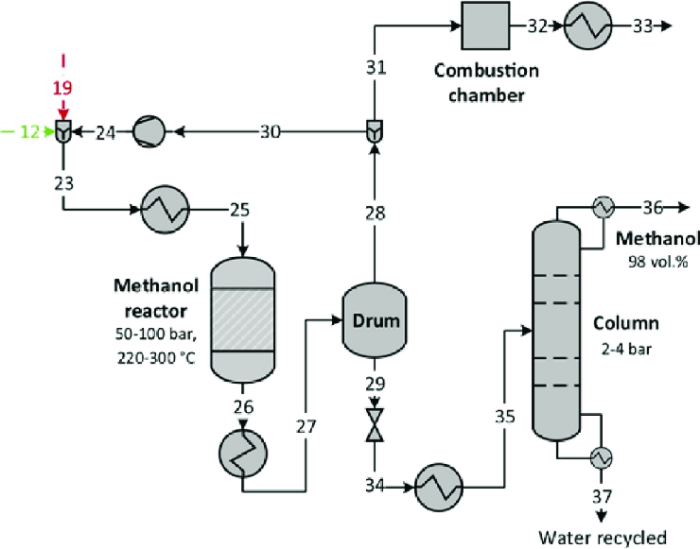
Types of Reactors
The most common types of reactors used for methanol synthesis are fixed-bed reactors and fluidized-bed reactors. Fixed-bed reactors are packed with catalyst pellets, while fluidized-bed reactors use a suspended catalyst in a fluidized bed of gas.
Design Considerations for Reactor Performance
- Catalyst loading and distribution
- Temperature and pressure gradients
- Gas flow distribution and mixing
- Reactor size and configuration
Operating Conditions for Methanol Synthesis
The operating conditions for methanol synthesis include a temperature range of 200-300°C, a pressure range of 50-100 bar, and a feed composition of CO:H2 = 1:2.
4. Thermodynamics and Kinetics of Methanol Synthesis

Thermodynamic Analysis
The methanol synthesis reaction is exothermic, with a negative change in enthalpy. The equilibrium constant for the reaction is a function of temperature and pressure, and the equilibrium conversion of CO to methanol increases with increasing pressure and decreasing temperature.
Kinetics of the Reaction, The synthesis of ch3oh from co and h2
The kinetics of the methanol synthesis reaction are complex and involve a series of elementary steps. The rate-determining step is the hydrogenation of CO to formaldehyde, which is followed by the addition of another hydrogen molecule to form methanol.
Effect of Temperature and Pressure on Reaction Equilibrium
Temperature and pressure have a significant impact on the reaction equilibrium. Increasing temperature shifts the equilibrium towards the reactants, while increasing pressure shifts the equilibrium towards the products.
5. Process Integration and Optimization
Integration with Other Chemical Processes
Methanol synthesis can be integrated with other chemical processes, such as coal gasification and natural gas reforming, to utilize waste gases and improve overall process efficiency.
Opportunities for Process Optimization
- Catalyst development and optimization
- Reactor design and operating conditions optimization
- Process integration and heat recovery
- Use of process simulation tools for process design and optimization
6. Applications of Methanol: The Synthesis Of Ch3oh From Co And H2

Major Applications of Methanol
- Fuel:Methanol is used as a fuel in transportation and power generation.
- Chemical feedstock:Methanol is a versatile chemical feedstock used in the production of a wide range of chemicals, including formaldehyde, acetic acid, and MTBE.
- Solvent:Methanol is used as a solvent in various industries, including the pharmaceutical, cosmetic, and paint industries.
Environmental and Economic Implications
The production and use of methanol have both environmental and economic implications. Methanol production can contribute to greenhouse gas emissions, while its use as a fuel can reduce air pollution. The economic viability of methanol production depends on factors such as the availability of feedstocks, the cost of catalysts, and the market demand for methanol.
Frequently Asked Questions
What is the primary application of methanol?
Methanol is primarily used as a chemical feedstock in the production of formaldehyde, acetic acid, and other chemicals. It is also employed as a fuel, particularly in the transportation sector, and as a solvent in various industries.
What factors influence the efficiency of methanol synthesis?
The efficiency of methanol synthesis is influenced by several factors, including the type of catalyst, reaction temperature and pressure, and the presence of promoters and support materials.
What are the environmental implications of methanol production?
Methanol production can contribute to greenhouse gas emissions if fossil fuels are used as the feedstock. However, advancements in renewable energy sources and carbon capture technologies offer opportunities for more sustainable methanol production.